When a malignancy or chronic infection sets in, a kind of immune combat fatigue can follow. Finding ways to recharge immune cells can restore their ability to fight deadly diseases, says immunologist John Wherry.
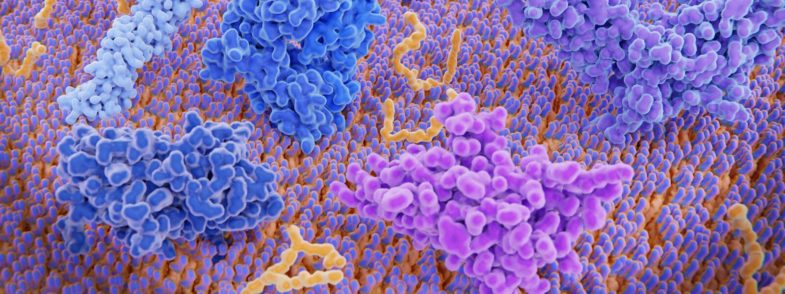
Understanding how cancer cells evade the immune system has led to new ways to kickstart T cells into helping battle tumors. One approach is to block the PD-1 receptor (shown in magenta on the surface of a T cell in this false-color rendering), which both healthy cells and wily cancer cells use to quash T cell attacks. CREDIT: JUAN GAERTNER / SHUTTERSTOCK
The human immune system has the power to fight off a vast array of viruses, infections and other pathogens. Yet when cancer strikes, that often isn’t enough. In some forms of the disease, tumor cells hide in plain sight, evading a patient’s T cells — parts of the immune system key to destroying pathogens. If a cancer evades them for long enough, the immune cells enter a state called “T cell exhaustion,” where those powerful defenders call off their attack, and linger in the background.
John Wherry, an immunologist at the University of Pennsylvania, thinks a promising type of immunotherapy (a family of treatments that manipulate the body’s own immune system to fight disease) could reverse this exhaustion, helping patients with melanoma and other similar cancers. In those diseases, tumors hide from the body’s T cells by attaching a molecule called PD-L1 onto their surface. The T cells sniff out this molecule to distinguish friend from foe: If they encounter a cell with PD-L1 on its surface, they ignore it and move on without a fight. Cells without PD-L1 aren’t so lucky — the T cells immediately attack and destroy them.
T cells detect these friend-or-foe molecules via a lock-and-key receptor called PD-1. Blocking PD-1 can spur the T cells to attack invaders with renewed vigor. Treatments based on this mechanism have turned cancers that were a death sentence into curable diseases — but it’s far from a perfect system and many questions remain. Knowable Magazine spoke with Wherry, coauthor of an article on exhausted T cells in the 2019 Annual Review of Immunology, about how reinvigorating spent T cells can help treat cancer, save the lives of patients and eventually lead to new ways to harness the immune system to treat disease. This conversation has been edited for length and clarity.
What usually happens in a healthy immune response, when the body recognizes a virus or cancer?
In a normal immune response, T cells and other cells of the immune system make a bunch of molecules that can destroy cancer cells or cells infected by a virus. As those T cells get activated, they divide incredibly fast, going from just a few hundred cells to literally tens of millions of cells in six or seven days. Now, this immune activity makes you feel pretty lousy, and can actually become dangerous. In massive influenza pandemics, like the one that happened in 1918–19, patients’ immune systems caused really severe tissue pathology, and that’s part of what killed them. The challenge is that your body needs to be able to mount an effective immune response against whatever the invading germ is, but not one that’s so effective that it destroys its own host tissues.
So what role does T cell exhaustion play in that process?
T cell exhaustion is a natural phenomenon, and it probably plays some important role in maintaining your overall health. It may be one way the immune system reaches a compromise between trying to kill a tumor or pathogen and not damaging your body.
Within a week or 10 days after the initial immune response, the process gets automatically shut off almost irrespective of whether you’ve cured the infection or not. The cells start to slow down in their division rate, and they start to die off to return to the body’s normal resting state. Now, when this all works well, the infection is wiped out before the immune system stands down, so there’s nothing to worry about. But if it hasn’t been wiped out, and the offending signals or the offending events that activated the immune system are still there, you wind up in this sort of stalemate. The T cells are getting a signal to be activated due to the infection, but they’re also getting signals to stand down, because otherwise your tissues may be damaged so badly that you could die. There’s kind of a chronic battle going on, and T cell exhaustion is the middle ground.
Does the immune system still stay active on some level, even though its T cells are standing down?
Yes. The immune system is constantly recognizing tumors and playing some role in limiting how fast they progress. If T cell exhaustion sets in, as it often does in certain cancers or chronic infections, you’re on this sort of balance point — your immune system is still fighting against the tumor, but not doing it well enough to completely get rid of it, and simultaneously, the tumor is growing and changing. Now, in some cases, if you change that balance a little bit, you can tip the scales for a patient. When the immune system stays suppressed, the tumor has the advantage. But if you can boost the activity of T cells or throw a little fuel on the fire, you may be able to fight back against the tumor.
T cells respond extremely differently to short-term (acute) and long-term (chronic) infections. In acute infections, T cell response spikes, then diminishes as the infection disappears, remaining prepared for a future encounter in the form of memory T cells. If the infection turns chronic (and thus the pathogen level or antigen load remains elevated), however, the immune cells stay partially activated, but are unable to fight off invading pathogens. These “exhausted” T cells can be revived with some immunotherapies.
So how do you go about reactivating T cells once they’re exhausted? And how can that help treat cancer patients?
One way would be by blocking the signals that tell them to stand down. Many tumor cells can evade the immune system because they’ve exploited the ability to make a molecule called PD-L1. Under normal conditions, only healthy cells in the body make this molecule. And the molecule tells T cells to ignore these healthy cells, since they’re on the same side. But if you block the receptor for PD-L1 on the T cell — what’s called the PD-1 receptor — the T cell can suddenly recognize the cancer as an invader and attack it. There are a few existing drugs on the market, like Keytruda or Opdivo, that can do this.
Fortunately, even if you block that PD-1 receptor, your immune system still usually avoids healthy cells, since they use a variety of other molecules to flag themselves as non-invaders. The immune system is really built on the idea of redundancy. If you had a single molecule responsible for keeping the system in check, and that molecule failed, it would be a life-threatening event. Yet even with this redundancy, there can be problems. In a subset of patients, the drugs that target PD-1 do result in the immune system attacking normal, healthy cells and causing side effects. Sometimes these side effects can be managed, but they often limit our ability to continue blocking PD-1 and treating the cancer. This is one of the reasons that we still need to do more research.
Healthy cells wave a white flag at the immune system by displaying a molecule called PD-L1. T cells detect that signal via a protein called PD-1, which binds to PD-L1 and keeps a T cell from attacking the body’s own cells. Some cancer cells trick the immune system by covering themselves in PD-L1, effectively cloaking them from view. Inhibitor drugs block that process, revealing the cancer so that the immune system can attack and destroy it.
You mentioned that some drugs already target PD-1 as a treatment. How well do they work?
In some cases, extremely well, but success rates still vary from patient to patient. The disease I work on, melanoma, has seen a massive change in treatment thanks to PD-1 inhibitor drugs. A decade ago, the expectation for stage IV melanoma patients was about six months to live. It was a real death sentence. Then PD-1 drugs arrived. Clinicians never used to use the word “cure” when they talked about melanoma, but we’re now seeing effective cures in about a third of our advanced-stage melanoma patients. That’s remarkable, transformational, but it still leaves two-thirds of patients who are not getting cured.
Why are we seeing only a 30 percent success rate? What needs to improve to make these drugs more effective?
The reasons that those patients aren’t getting cured varies widely and we still don’t understand this problem fully. Some people may not get enough reinvigoration of their exhausted T cells. Some may not have had a T cell response to their cancer to begin with, which is often the case in diseases like pancreatic cancer. It’s much more difficult to convince the immune system to fight a cancer if it never started on its own. In other cases, the immune response did everything it was supposed to do, and the tumor mutated, so now the immune system can’t recognize it anymore. In a case like that, a patient can take anti-PD-1 drugs, have a pretty good response where the immune system fights their tumor, and all of a sudden the tumor mutates and the treatment stops working.
Those are three of the big classes of problems that we still have to address, and each of those affects a huge number of cancer patients. The proportions may differ depending on whether you’re talking about melanoma or lung cancer or colorectal cancer, but each of these cases needs more research and clinical trials. So despite this really truly transformational advance in medicine, there are still many questions to answer and we still have a long way to go.

An exhausted T cell. In June, Omar Khan, John Wherry and others published a paper in Nature linking the presence of a protein, TOX (stained blue), with the T cell exhausted state. Pink shows DNA, while white highlights the cell surface. CREDIT: JOHN WHERRY / PENN MEDICINE
Are there cancers that don’t respond to these sorts of drugs?
Yes. PD-1 drugs are really only effective for cancers that trigger an immune response. Most melanomas arise from ultraviolet radiation-induced mutations, and as far as the immune system is concerned, that makes them look as foreign as a virus or bacterium. So when you have tumors that have a lot of mutations, they look really foreign, and the immune system triggers its “on” switch. But in other tumors, like in pancreatic cancer, some types of brain cancer, some sarcomas, there are very few mutations, and the immune system doesn’t have a strong “on” switch. In order to treat those with immunotherapy, you would need to make sure that the immune system has some way to recognize the tumor.
Are there other diseases besides cancer that could benefit from reversing T cell exhaustion?
Well, chronic infectious disease is a big one. There are trials in HIV and hepatitis infections to give anti–PD-1 drugs, and there’s some talk about malaria as well. Autoimmunity would be another big one. For those patients, you’d want to go in and activate the PD-1 pathway instead of blocking it to slow down an immune response.
There’s a growing body of evidence that pathways like PD-1 play a role in many other diseases, however, like Alzheimer’s and other neurodegenerative disease. There’s some evidence perhaps that it could work for cardiovascular disease as well.
What do these treatments mean for the future of medicine? Could you see a future where we can treat chronic disease by just manipulating our immune systems?
I think that’s exactly the aspirational goal. It’s going to take decades to get there, but that’s why things are so exciting right now. If we truly understand how to tune the dial for the immune system, then what we’re doing in cancer opens the door to treat other diseases as well. We’re now giving drugs that are essentially retraining and reprogramming how the immune system works. If you fast forward and take what’s happening to its logical extension, that’s the goal: to really think about how we can make precision immune therapy part of our normal clinical operation.